TALVEYTM (talquetamab-tgvs), a first-in-class bispecific antibody for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody, has been granted accelerated approval by the U.S. Food and Drug Administration (FDA).
TALVEY™ is available only through a restricted program called the TECVAYLI® and TALVEY™ Risk Evaluation and Mitigation Strategy (REMS).
TALVEY approval is based on the response rate and durability of response based on the Phase I/II MonumenTAL-1 study (Phase I: NCT03399799; Phase II: NCT04634552),which included over 300 individuals in a single-arm, open-label, multicohort, multicenter dose-escalation study.
Phase I evaluated the safety and efficacy of TALVEY™ in adults with relapsed or refractory multiple myeloma who received three or more prior lines of therapy.
Phase II of the study evaluated the efficacy of TALVEY™ in participants with relapsed or refractory multiple myeloma at the recommended Phase 2 dose(s) (RP2Ds), established at SC 0.4 mg/kg weekly and 0.8 mg/kg every two weeks, respectively.
Mechanism of Action of TALVEYTM:
TALVEYTM, a bispecific T-cell engaging antibody binds to the CD3 receptor on T cells as well as the G protein-coupled receptor class C group 5 member D (GPRC5D) expressed on the surface of multiple myeloma cells, benign plasma cells, and healthy tissue like epithelial cells in two keratinized tissues of the skin and tongue.
“The clinically meaningful efficacy and safety profile observed with talquetamab in heavily pretreated patients in this clinical trial, which included patients treated with prior BCMA-targeted bispecific or CAR-T cell therapy, has been notable,” – said Ajai Chari, M.D., Director of Multiple Myeloma Program, Professor of Clinical Medicine at the University of California, San Francisco. “Patients at this stage of disease have a poor prognosis. Talquetamab as a first-in-class therapy is a new option for patients with this difficult-to-treat blood cancer.”
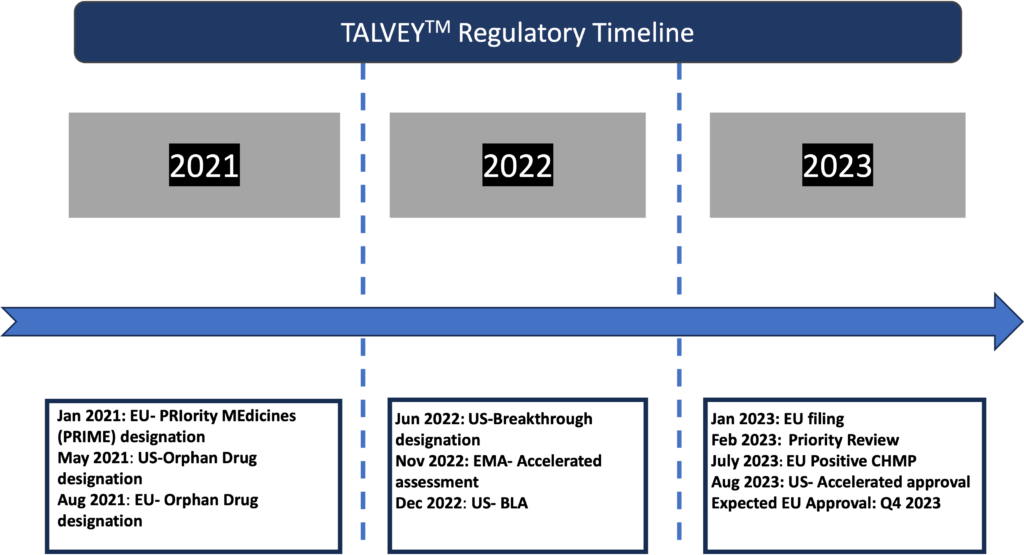
Regulatory Timeline Of TALVEYTM Approval:
The U.S. FDA and the European Commission, respectively, granted TALVEYTM Orphan Drug Designation in May 2021 and August 2021 for the management of multiple myeloma.
In June 2022, the U.S. FDA also designated TALVEYTM as a breakthrough therapy for the treatment of adult patients with relapsed or refractory multiple myeloma who have already received at least four prior lines of therapy, including proteasome inhibitors, immunomodulatory drugs, and anti-CD38 antibodies.
The FDA decided in February 2023 to start a Priority Review of the Biologics Licence Application (BLA) submitted in December 2022, and this decision was followed by the approval.
Other Clinical trials involving TALVEYTM:
Talvey is currently been investigated in combination and in sequence across all lines of multiple myeloma in studies with other bispecific antibodies as well as with existing standards of care.
| S.No | Clinical Trial title | Phase | NCT ID | Trial Name |
| 1 | A Study of the Combination of Talquetamab and Teclistamab in Participants With Relapsed or Refractory Multiple Myeloma. | I/II | NCT04586426 | RedirecTT-1 |
| 2 | A Study of Subcutaneous Daratumumab Regimens in Combination With Bispecific T Cell Redirection Antibodies for the Treatment of Participants With Multiple Myeloma | I | NCT04108195 | NA |
| 3 | A Study of Talquetamab With Other Anticancer Therapies in Participants With Multiple Myeloma | I/II | NCT05050097 | MonumenTAL-2 |
| 4 | A Study of Talquetamab and Teclistamab Each in Combination With a Programmed Cell Death Receptor-1 (PD-1) Inhibitor for the Treatment of Participants With Relapsed or Refractory Multiple Myeloma | I | NCT05338775 | TRIMM-3 |
| 5 | A Study Comparing Talquetamab in Combination With Daratumumab or in Combination With Daratumumab and Pomalidomide Versus Daratumumab in Combination With Pomalidomide and Dexamethasone in Participants With Multiple Myeloma That Returns After Treatment or is Resistant to Treatment | III | NCT05455320 | MonumenTAL-3 |


Nice information