Eli Lilly and Company (NYSE: LLY) announced that today on 14-August-23, its purchase of Sigilon Therapeutics, Inc. (NASDAQ: SGTX) was completed successfully. Lilly will be able to continue investigating and developing encapsulated cell treatments, including SIG-002, for the treatment of type 1 diabetes as a result of the acquisition.
“Make life better – that’s the phrase that guides everything we do at Lilly,” said Ruth Gimeno, Ph.D., group vice president, diabetes, obesity and cardiometabolic research at Lilly. “We are excited to welcome our new colleagues from Sigilon to Lilly; together, we will strive to provide solutions for people living with type 1 diabetes that absolves them of constant disease management, and advance Sigilon’s technology for patients.”
As previously announced, Lilly and Sigilon entered into a Merger Agreement on June 28, 2023, and on July 13, 2023, Lilly and a wholly owned subsidiary (“Purchaser”) launched a tender offer (the “Offer”) to purchase all of Sigilon’s issued and outstanding shares (“Shares”) in exchange for:
(a) $14.92 per Share in cash, without interest (the “Cash Consideration”) and less any applicable tax withholding.
(b) one non-tradable contingent value right (“CVR” and, together with the Cash Consideration, the “Offer Price”) per Share, representing the contractual right to receive contingent payments of up to $111.64 per Share in cash, net to the stockholder in cash, without interest, and less any applicable tax withholding.
There is no guarantee that any payments will be made in relation to the CVRs. The Offer terminated on August 9, 2023, as planned, with 1,718,493 Shares validly tendered and not properly withdrawn, representing 76.61% of the issued and existing Shares, combined with Shares previously owned by Lilly. Purchaser accepted for payment all such validly tendered and not lawfully withdrawn Shares in accordance with the terms of the Offer.
Following the acceptance of the Offer, Lilly completed its acquisition of Sigilon on August 11, 2023, through the merger of Purchaser with and into Sigilon in accordance with Section 251(h) of the General Corporation Law of the State of Delaware), with Sigilon surviving such merger as a wholly owned subsidiary of Lilly.
In connection with the merger, each Share issued and outstanding immediately prior to the effective time of the merger (other than (i) Shares held in Sigilon’s treasury or owned by Sigilon, or owned by Lilly, Purchaser or any direct or indirect wholly-owned subsidiary of Lilly or Purchaser or (ii) Shares held by any stockholder of Sigilon who was entitled to demand and properly demanded appraisal for such Shares in accordance with Section 262 of the DGCL), including each Share that was subject to vesting or forfeiture restrictions granted pursuant to a Sigilon equity incentive plan, program or arrangement, was canceled and converted into the right to receive the Offer Price, without interest, less any applicable tax withholding.
The NASDAQ Global Select Market has delisted Sigilon’s common stock, and the company will be deregistered under the Securities Exchange Act of 1934, as amended.
Morgan, Lewis & Bockius LLP is providing as legal counsel for Lilly. Lazard is the principal financial advisor for Sigilon, and Ropes & Grey LLP is the legal counsel. Canaccord Genuity also served as Sigilon’s financial advisor.
Lilly Acquired Sigilon Therapeutics’ Technology platform:
For many years, researchers have investigated cell and gene therapies as potential replacement therapies for severe acute and chronic diseases. Despite major advancements in the field, these approaches carry a number of limitations including immune rejection, limited eligibility, durability and variability challenges, inability to re-dose and high manufacturing costs. To address these drawbacks, Sigilon has developed novel Shielded Living Therapeutics™ (SLTx) platform, which is expected to have the potential to result in functional cures for patients with a wide range of acute and chronic diseases.
Shielded Living Therapeutics™ (SLTx) platform:
The products candidates themselves are generated through three common elements: the cell, the sphere, and our streamlined manufacturing process.
The Cell:
- High levels of desired proteins, enzymes, or medicinal compounds are expressed.
- Proteins are delivered at a fixed rate or are engineered to “sense and respond” to changes in the environment.
- Survive in an encapsulation environment well
- Large-scale production is possible.
- Are comparable to cells already utilised in clinical trials
- Allow for the simple addition of new gene expression cassettes for various applications.
The Sphere:
The sphere is made of our patented AfibromerTM matrix, which has been engineered to be excellent for housing thousands of therapeutic cells. Sigilon created this dual-layer sphere to prevent or minimise immunological rejection based on basic insights made at MIT.
1) Alginate spherical outer layer with AfibromerTM
• Novel small chemical targeted to suppress immune response
• Allowing nutrient ingress and protein outflow
2) Optimised for viability and productivity in the inner section
Manufacturing:
Sigilon has developed a cutting-edge manufacturing platform that is modular for all prospective product options. Our manufacturing approach is designed for reproducibility, speed, and cost-efficiency, with the goal of offering a real “off-the-shelf” solution for patients, with almost all parts of the platform common across research programmes.
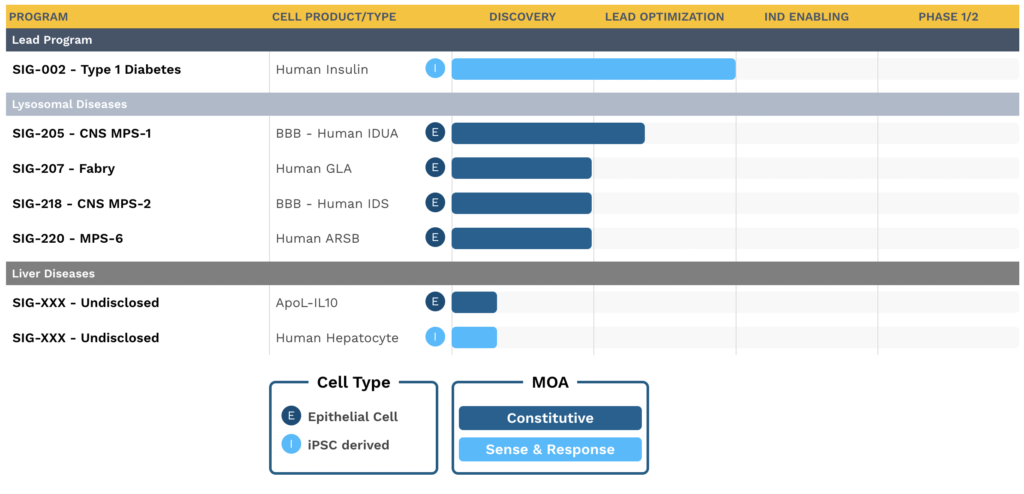
Lilly Acquired Sigilon Therapeutics’ Pipeline:
The company’s pipeline predominantly consists of endocrine and lysosomal illnesses, with the present emphasis on diabetes. product candidates are intended to be off-the-shelf, long-lasting, controlled, and redosable without requiring gene alteration or persistent immune suppression of the patient.
About Lilly:
Lilly unites caring with discovery to create medicines that make life better for people around the world. We’ve been pioneering life-changing discoveries for nearly 150 years, and today our medicines help more than 51 million people across the globe.
Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world’s most significant health challenges, redefining diabetes care, treating obesity and curtailing its most devastating long-term effects, advancing the fight against Alzheimer’s disease, providing solutions to some of the most debilitating immune system disorders, and transforming the most difficult-to-treat cancers into manageable diseases.
With each step toward a healthier world, we’re motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable.

