Sandoz, a global pioneer in generic and biosimilar pharmaceuticals, said today that the US Food and Drug Administration (FDA) has approved Polpharma Biologics’ biosimilar Tyruko® (natalizumab-sztn). Tyruko is the first and only FDA-approved biosimilar for relapsing forms of multiple sclerosis (MS) and is approved to treat all indications covered by the reference drug.
Tyruko is approved as a monotherapy in adults for all indications covered by reference drug Tysabri® (natalizumab), including clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), and active secondary progressive illness, as well as Crohn’s disease.
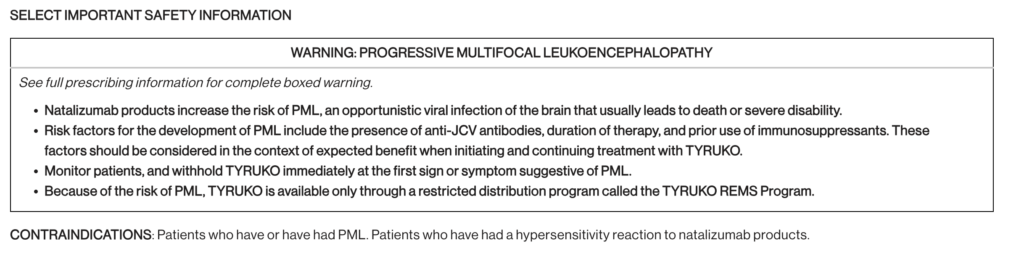
The FDA granted approval based on a comprehensive data package that included analytical, functional, and clinical information. The approval comes with safety warning labelling and a Risk Evaluation and Mitigation Strategy (REMS).
Tyruko is administered intravenously (IV) and has the same dose form, method of administration, dosing regimen, and presentation as the reference drug. Sandoz is committed to all elements of patient safety with Tyruko, which will be available through the Sandoz REMS programme at launch.
Biosimilar Tyruko Developmental History:
In 2019, Sandoz and Polpharma Biologics signed a global marketing deal for Tyruko. Under the terms of agreement, Polpharma Biologics shall retain responsibility for the active substance’s development, production, and supply. On the other hand, Sandoz owns the unique global licence to commercialise and distribute it in all markets. Sandoz is committed to providing this critical medicine to patients in the United States as quickly as feasible.
Expert Opinion on Biosimilar Tyruko Approval:
Keren Haruvi, President North America, Sandoz Inc., said: “Of the nearly one million people in the US living with multiple sclerosis, hundreds of thousands experience disease relapse. Tyruko has the potential to extend the reach of natalizumab treatment for these patients, increase healthcare savings and fuel innovation through competition in the market.”
Bari Talente, the National MS Society’s Executive Vice President for Advocacy and Healthcare Access, said: “Access to affordable, high-quality healthcare is essential for people with multiple sclerosis to live their best lives. The approval of Tyruko, the first FDA-approved biosimilar disease-modifying treatment for people with relapsing forms of MS, is a milestone. Biosimilars are an important treatment option because they have no clinically meaningful differences from their reference medicines. Prescribing them can increase accessibility to affordable medications, improve adherence and help contain healthcare costs.”
Multiple Sclerosis:
MS is a chronic inflammatory and neurodegenerative illness of the central nervous system that has a significant impact on daily living. Most persons with MS have periods when they have new symptoms or relapses that improve partially or fully, followed by periods when they are disease-free.
About Biosimilar Tyruko® (natalizumab-sztn):
Tyruko was designed to be very close to the reference drug, a well-established, extremely effective anti-4 integrin monoclonal antibody disease-modifying treatment in relapse types of multiple sclerosis (MS). Tyruko is approved as a monotherapy in the United States for relapsing types of multiple sclerosis (MS), including clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), and active secondary progressive disease, as well as Crohn’s disease in adults. It is the first and only biosimilar approved by the FDA for relapsing types of MS.
Biosimilar Tyruko® (natalizumab-sztn)- Indications:
Multiple Sclerosis (MS):
Biosimilar Tyruko is approved as a single treatment for individuals with relapsing types of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive illness. Products containing natalizumab increase the risk of progressive multifocal leukoencephalopathy (PML). Natalizumab is thus only available through specific Risk Evaluation and Mitigation Strategy (REMS) programmes. When starting and continuing treatment with Biosimilar Tyruko, physicians should examine if the predicted benefit outweighs the risk.
Crohn’s Disease (CD):
Biosimilar Tyruko is recommended for initiating and maintaining clinical response and remission in adult patients with moderately to highly active Crohn’s disease who have had an unsatisfactory response to or are unable to tolerate standard CD treatments including TNF-inhibitors. TYRUKO should not be used with immunosuppressants (such as 6-mercaptopurine, azathioprine, cyclosporine, or methotrexate) or TNF-inhibitors.